Introduction
The containment of a core material inside of a small capsule is called microencapsulation. A polymeric material coates liquid or solid substances to protect polymeric material from circumambient area1. Microcapsules size vary between 50 nm to 2 mm2. Microcapsule’s size and structure differs according to core material being solid, liquid or gas as in figure 12.
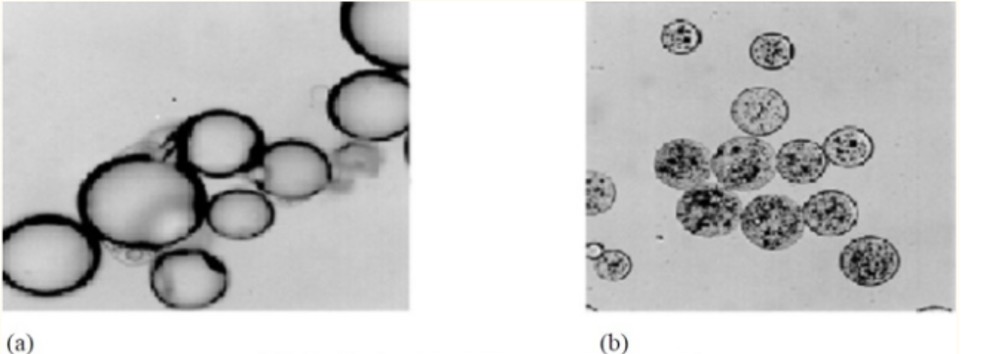
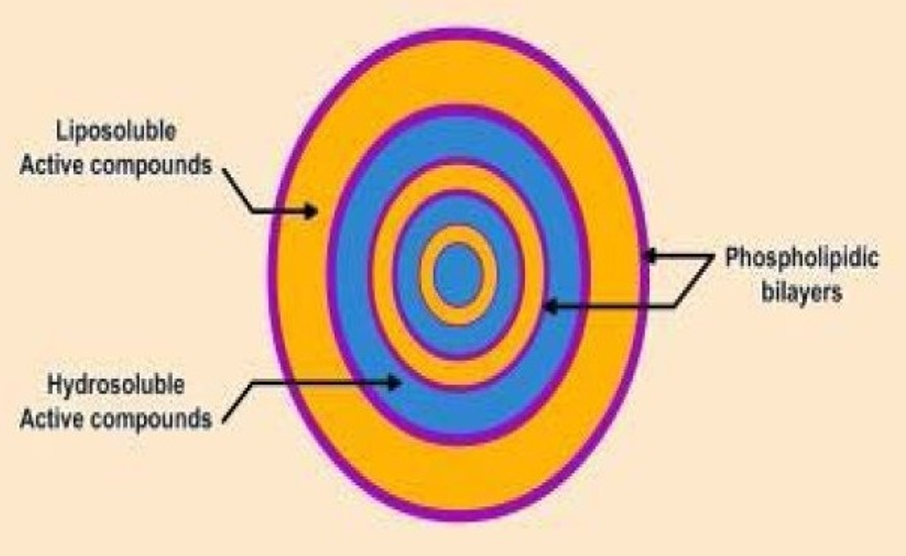
Coating material must be adhesive to the core material in order to cover core material properly. Coating materials must work as an harmonious aid to core material in required strength, flexibility, impermeability, optical properties, and stability. Its release must be controllable under required conditions1.
Figure 3 : Coating material examples1
| Water Soluble Materials | Water Insoluble Materials | Waxes and Lipid Materials |
| Gelatin | Calcium alginate | Paraffin |
| Gum Arabic | Polyethylene | Carnauba |
| Starch | Polyamide (Nylon) | Spermaceti |
| Polyvinylpyrrolidone | Silicones | Beeswax |
| Polyacrylic acid | Polymethacrylate | Stearic acid |
| Carboxymethyl-cellulose | Cellulose nitrate | Glyceryl stearates |
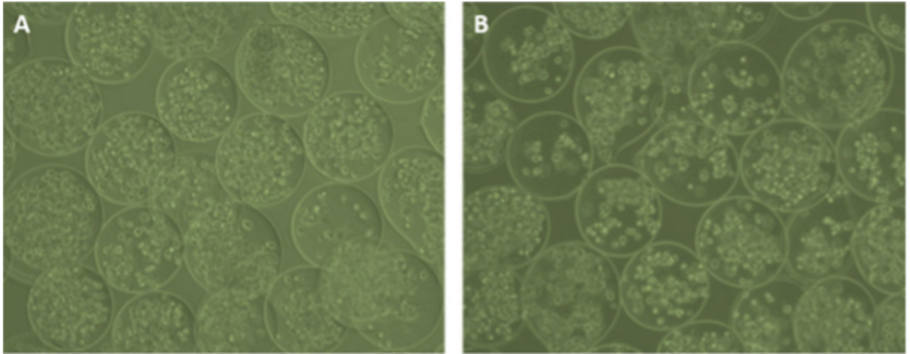
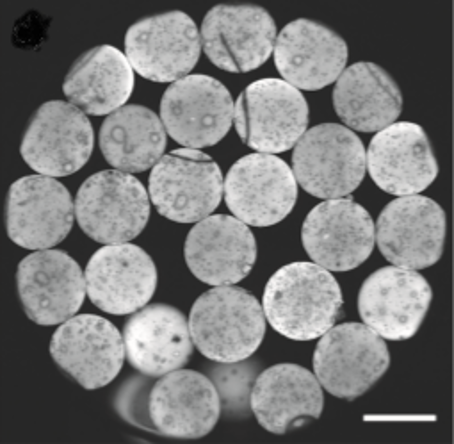
Figure 5 : Confocal laser scanning microscope image of rhodamine-labeled hydrogel microcapsules4.
Method
The microencapsulation of adipose stem cells coating with alginate is shown in figure 6. The cross- linking solution contains calcium chloride and glucose and is buffered with HEPES. Calcium chloride provides divalent cations to alginate during cross-linking. Glucose is useful for maintaining physiological osmolality of the cross-linking solution for the adipose stem cells. HEPES is used tomaintain pH at or below pH 7.33.
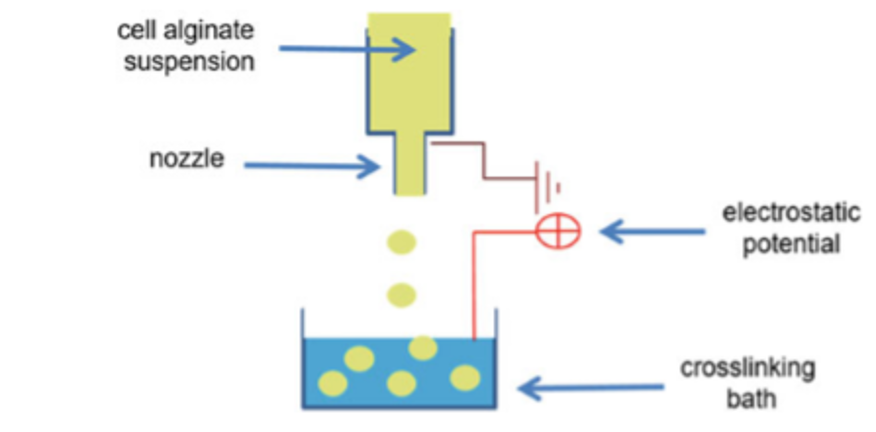
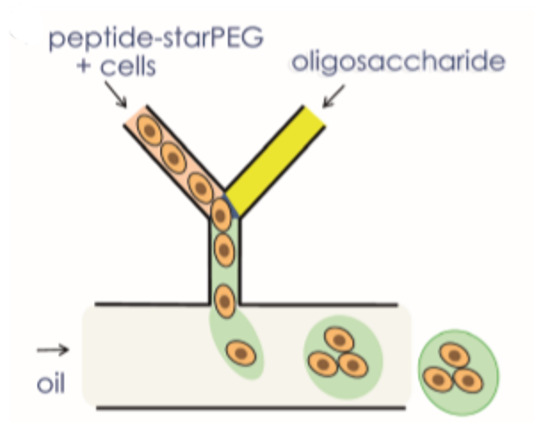
The generation of hydrogel microcapsules with a microfluidic system is shown in figure 7. Oligosaccharides and peptide–starPEG were inserted through two distinct channels. The flow rates of the oil phase and Oligosaccharides and peptide–starPEG have been set to get required droplet formation4.
Conclusion
Microencapsulation can be used to encapsulate different materials therefore it is useful for treatment of different diseases that occurs in various tissues. There are various methods to make microcapsules. Microcapsule generation method must be chosen carefully according to the materials that microcapsule made out of. Microcapsules can be used to deliver drug molucules, various cell types into the targeted tissue. As technology improves, microencapsulation mehods will also improve and become more effective.
References
1. MICROENCAPSULATION. Int J Pharm Sci Rev Res. 2010;5(2):58-62.
2. M.N. Singh, K.S.Y. Hemant, M. Ram and HGS. Microencapsulation: A promising technique for controlled drug delivery. Res Pharm Sci. 2010;5(2):65-77.
3. Shirae K. Leslie , Ramsey C. Kinney , Zvi Schwartz and BDB, Abstract. Microencapsulation of Stem Cells for Therapy. In: Vol 1479. ; 2017:225-235. doi:10.1007/978-1-4939-6364-5
4. Wieduwild R, Krishnan S, Chwalek K, et al. Noncovalent Hydrogel Beads as Microcarriers for Cell Culture. Angew Chemie. 2015;127(13):4034-4038. doi:10.1002/ange.201411400
